- mycobacteria intrinsically resistant to most abx
- grow slowly - abx act on dividing cells
- can be dormant - completely resistant or killed very slowly
- lipid rich cell wall - impermeable to many agents
- intracellular pathogen - organism residing in macrophages are difficult to be entered
- development of resistance is notorious
ANTUBERCULOUS DRUGS
Introduction
- 1st line agents - RIPES
- I+R - 2 most active drugs - 9 months of both will cure 95-98% of cases
- addition of pyrazinamide to above for 1st 2 mths --> reduce duration to 6 mths
- ethambutol and streptomycin
- does not reduce duration of rx
- for coverage of resistant bugs while culture pending
- INH resistance in US 10%
- both INH and R resistance (MDR) 3%
- most active drug
- less effective for atypicals
- penetrates into macrophages --> extracellular and intracellular microbes
- MOA
- inhibit mycolic acid synthesis - components of mycobacterial cell wall - cell death
- drug resistance happens in 1 x 10*6
- TB lesions has 1 x 10*8 bacilli - resistant mutants are readily selected
- adding rifampicin - resistance 1 x 10*6 x 10*6 - much higher
- po - well absorbed
- penetrates all tissue including CNS
- metabolised by liver, renally excreted
- don't need to adjust dose in renal pt
- hepatitis - commonest, up 3-4x normal - dont need to stop drugs, if clinically symptoms occur (loss of appetite, n/v, jaundice, RUQ pain - stop drugs promptly (causes liver cell necrosis)
- peripheral neuropathy - due to relative pyridoxine deficiency (INH promotes pyridoxine excretion) - hence higher risk for alcoholic/malnutrition/DM/AIDS/uremia
- immunologic - rash, fever, SLE
- blood disorders - pyridoxine def anemia, tinnitus, GI discomfort
- semisynthetic abx of rifamycin, produced by Streptomyces mediterranei
- g+ve/-ve, enteric bacteria, mycobacteria (bactericidal), chlamydia
- readily penetrates tissues, phagocytes --> enters abscess and lung cavities
- MOA
- binds to B-subunit of RNA polymerase --> (-) RNA synthesis
- resistance - point mutation - reduction in binding to polymerase
- mycobacteria infection
- others - meningococcal carriage, Hib in kids, + fusidic acid = against staph carriage
- orange tinge to bodily fluid
- hepatitis
- allergic type - rash, fever, thrombocytopenia
- synthetic agent
- inhibits mycobacterial arabinosyl transferases --> needed for polymerization of arabinoglycan (component of cell wall)
- resistance - due to mutation unable to bind to transferases
- SE - retrobulbar neuritis - loss of visual acuity, red-green color blindness --> C/I in children too young to allow visual assessment
Pyrazinamide
- exact mechanism unknown
- use - important as 1st line agent to reduce rx time 6mths - as 'sterilizing' agent
- SE - liver toxicity, hyperuricemia (may provoke gout)
Streptomycin
- aminoglycoside - see chapter 45
- penetrate into cells poorly - mainly against extracellular tubercle bacilli
ALTERNATIVE 2ND LINE AGENTS
Introduction
- considered only
- resistance to 1st line
- failure of lcinical response
- serious drug reactions
- expert guidance to deal with the SE of the agents listed
- Ethionamide - related to INH - blocks mycolic acid
- Capreomycin - peptide protein synthesis inhibitor
- Cycloserine - inhibit cell wall synthesis
- Aminosalicylic acid (PAS) - anti-folate exclusively against TB
- Kanamycin & amikacin
- Fluoroquinolone
- Linezolid
- Rifabutin
- Rifapentine
- 10% of clinical practice in US - not M tuberculosis or M tuberculosis complex
- M avium complex - M avium, M intracellulare
- special lab characteristic
- present in environment
- not communicable btw person
- less susceptible to normal antiTB drugs
- M kansasii - INH, R, ethambutol
- M avium
- common - important in AIDS
- combination agents - azythro/clarithro + ethambutol +- ciprofloxacin
- M leprae
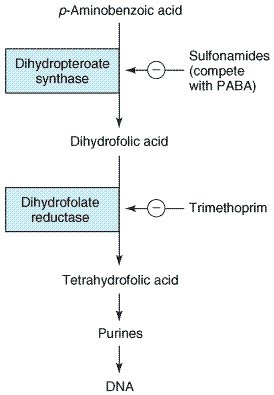
- dapsone + other sulfones
- closely related to sulfonamides - same MOA
- resistance issue - used in combination with 3 agents - dapsone, rifam, clofazimine
- NB: dapsone can be used for PCP
- SE: hemolysis (common, esp in thos with G6PD deficiency), metHb, erythema nodosum (can be rx with steroids/thalidomide)
- clofazimine
- phenazine dye - alternative to dapsone
- MOA unknown - DNA binding
- SE: skin discoloration - red brown to nearly black